ABOUT COLUMBUS
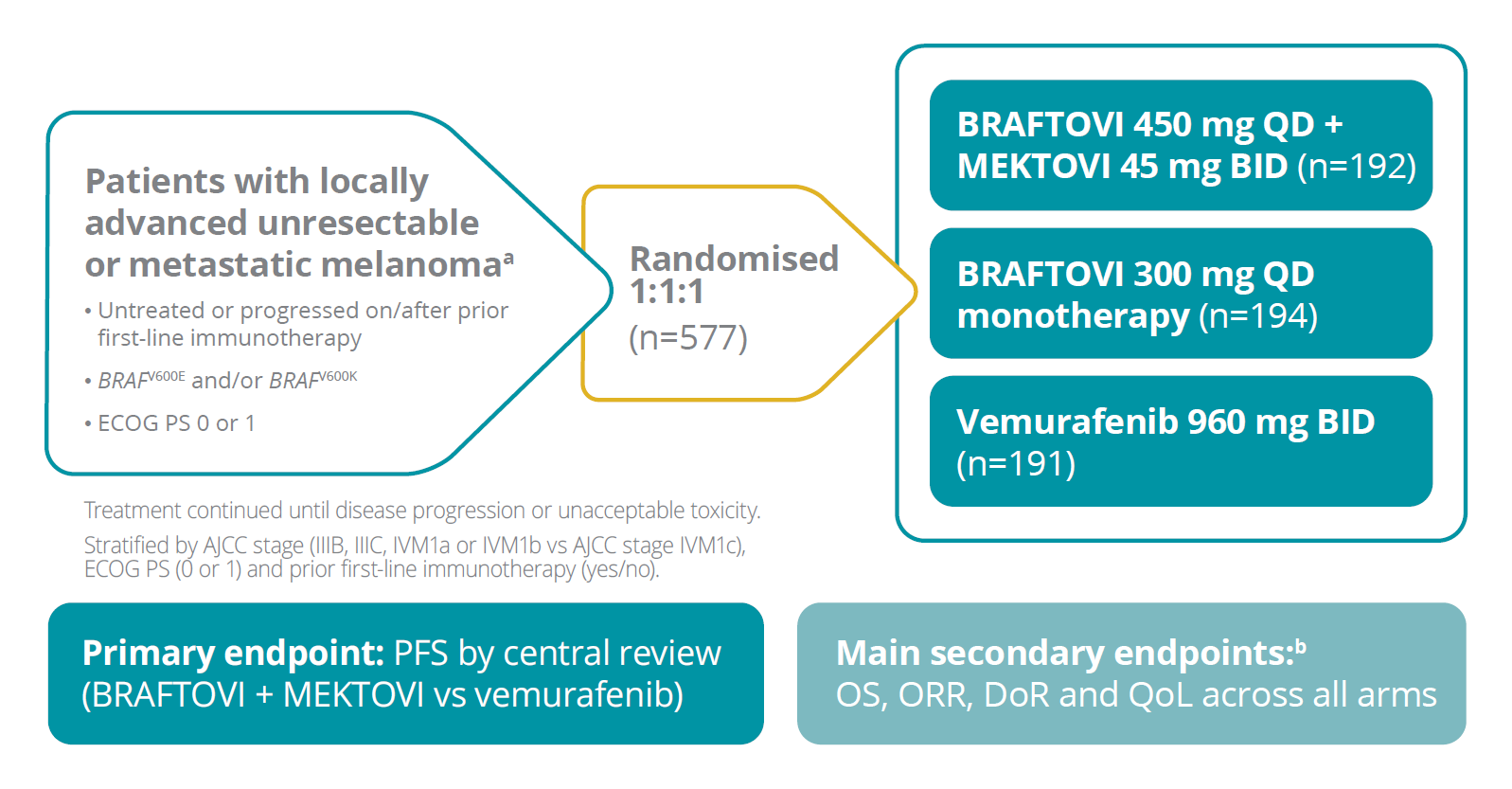
The COLUMBUS trial was a randomised, Phase 3, multicentre, open-label, active-controlled study5,6,16
Explore COLUMBUS efficacy results on the efficacy page
BRAFTOVI® + MEKTOVI® was studied in a broad range of patients5
95% of patients in COLUMBUS were treatment naïve for metastatic disease6
| Select baseline characteristics6 | BRAFTOVI® (450 mg) + MEKTOVI® (45 mg)a n=192 |
vemurafenib (960 mg) n=191 |
| Median age (range), years | 57 (20–89) | 56 (21–82) |
| ECOG PS 0, % | 71 | 73 |
| LDH ≥ULN, % | 29 | 27 |
| BRAFV600E/K mutation status, % | 89/11 | 88/12 |
| Tumour stage at study entry, % | ||
| IIIB/IIIC | 5 | 6 |
| IVM1a | 14 | 13 |
| IVM1b | 18 | 16 |
| IVM1c | 64 | 65 |
| Number of organs involved, % | ||
| 1 | 24 | 24 |
| 2 | 30 | 31 |
| ≥3 | 45 | 46 |
| Previous immunotherapy in advanced or metastatic setting, % | ||
| Ipilimumab | 3 | 3 |
| Anti-PD-1 or anti-PD-L1 | 1 | 0 |
| Interferons or interleukins | 2 | 3 |
a Dosing regimen for the combination was BRAFTOVI® 450 mg QD + MEKTOVI® 45 mg BID.6 b By American Joint Committee on Cancer (AJCC) 7th edition.
BID, twice daily; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; QD, once daily; ULN, upper limit of normal.