EFFICACY
Explore BRAFTOVI® + MEKTOVI® long-term efficacy data
Explore BRAFTOVI® + MEKTOVI® long-term efficacy data
PFS rate by central review %a,b,c

More than 1 in 5 patients remained progression-free at 7 years
with BRAFTOVI® + MEKTOVI® (21%) 3,f
a Curve adapted from SmPCs, Schadendorf et al. 2024, and Dummer et al. 2022.1-4 b PFS (central review) was the primary endpoint of the COLUMBUS study.5 In the initial analysis (cut-off date May 2016), BRAFTOVI® + MEKTOVI® achieved a median PFS of 14.9 months vs 7.3 months for vemurafenib (HR=0.54 [95% CI: 0.41–0.71] nominal p<0.0001).5 c Final analysis. Cut-off date: March 2023.1,2 d In progression or death.5 e Nominal p-value. f Cut-off date: January 2023.3 Results at 7 years are from a descriptive post-hoc analysis and should be interpreted in the context of this limitation.
CI, confidence interval; HR, hazard ratio; PFS, progression-free survival; LDH, lactate dehydrogenase.
Overall Survival rate %a,b,c
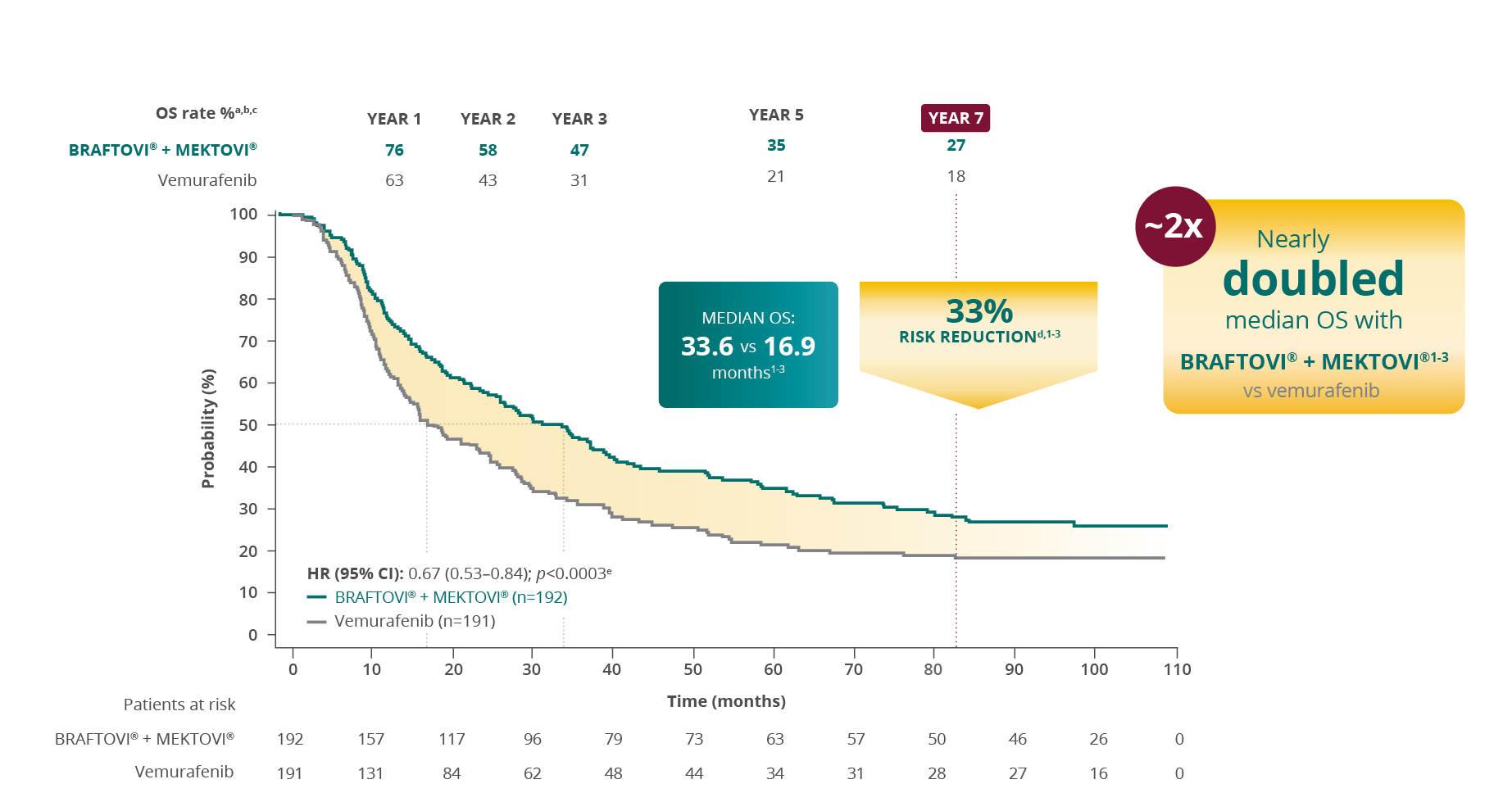
More than 1 in 4 patients were still alive at 7 years
with BRAFTOVI® + MEKTOVI® (27%)3,f
a Curve adapted from SmPCs and Schadendorf et al. 2024.1-3 b OS was a secondary endpoint of the COLUMBUS study with a nominal p value.6 c Cut-off date: March 2023.1,2 d In death.6 e Nominal p-value. f Cut-off date: January 2023.3 Results at 7 years are from a descriptive post-hoc analysis and should be interpreted in the context of this limitation.
CI, confidence interval; HR, hazard ratio; LDH, lactate dehydrogenase; OS, overall survival.
ORR by central reviewa,b,1,2
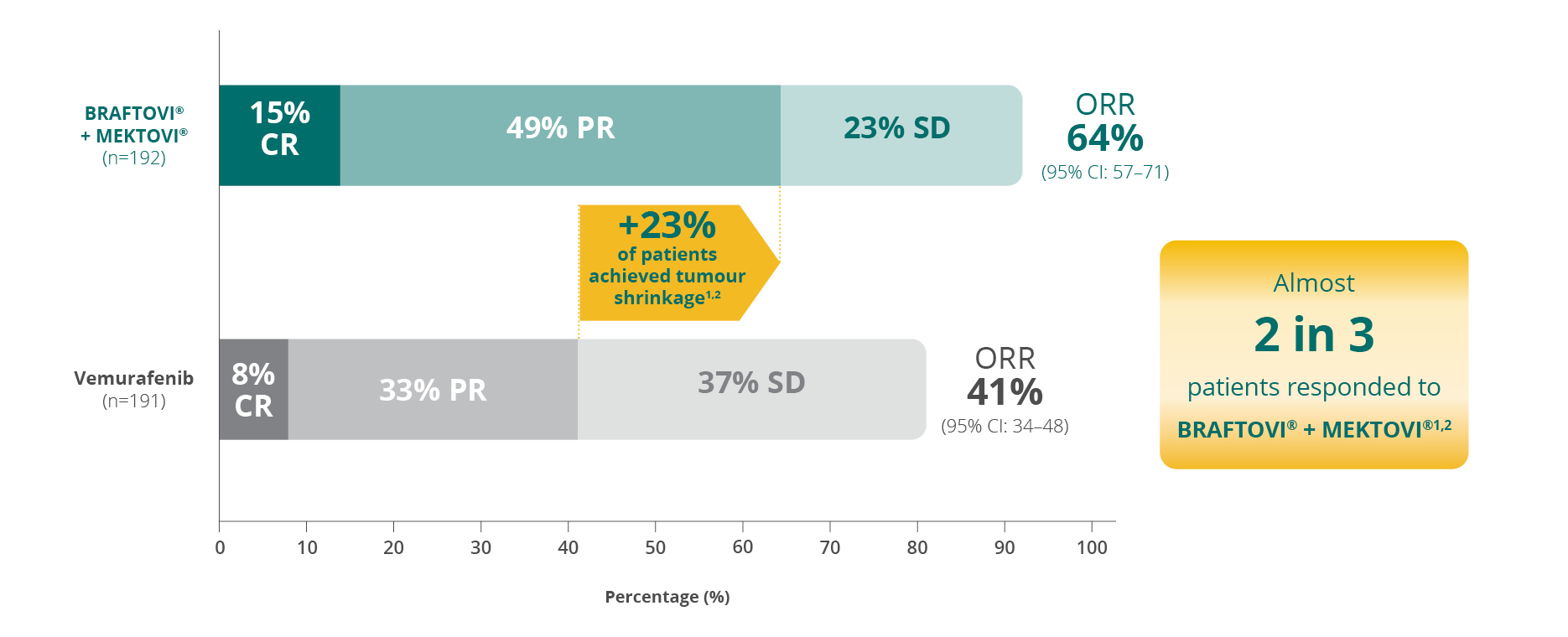
More than 9 in 10 patients achieved disease control
with BRAFTOVI® + MEKTOVI® (92% vs 81% for vemurafenib)c,d,e,1,2
Median DoR by central reviewa,b,1,2
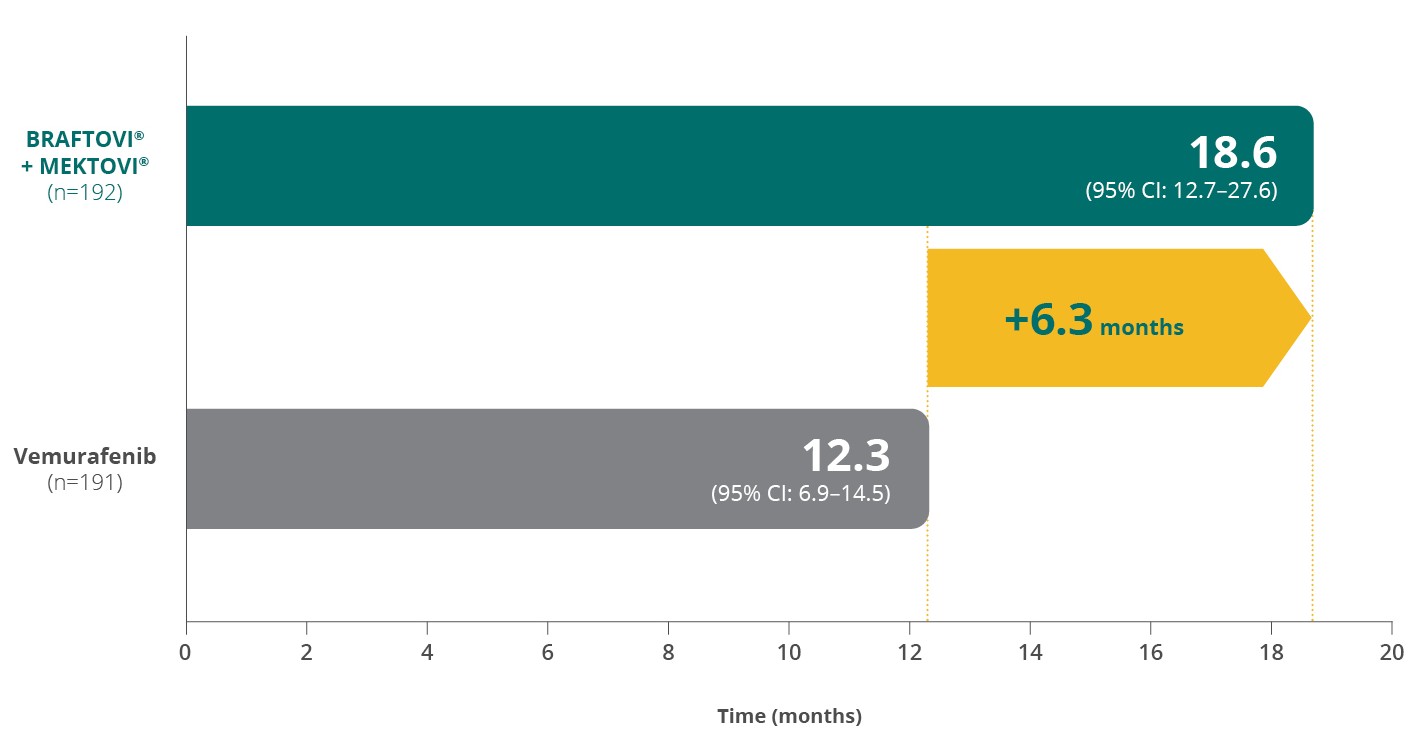
BRAFTOVI® + MEKTOVI® extended the median duration of response
by more than 6 months vs vemurafenibc,d,1,2
The 7-year analysis is post hoc and descriptive.
a Adapted from SmPCs.1,2 b ORR and DoR were secondary endpoints of the COLUMBUS study.6 c Final analysis. Cut-off date: March 2023. d By central review.1,2 e Disease control rate = CR+PR+SD+Non-CR/Non-PD.1,2
CI, confidence interval; CR, complete response; DoR, duration of response; ORR, overall response rate; PR, partial response; SD, stable disease
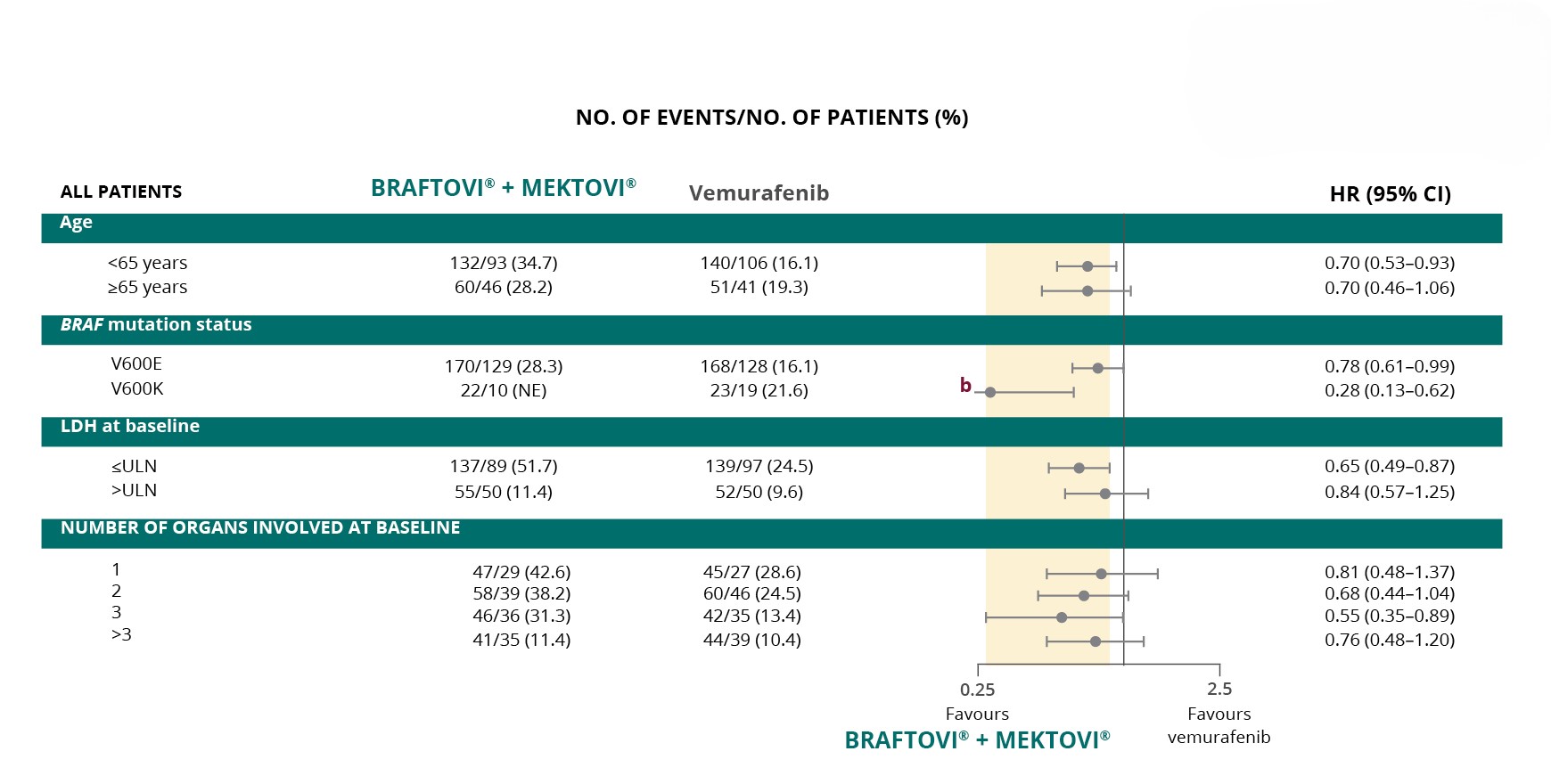
BRAFTOVI® + MEKTOVI® achieved better OS outcomes across
various patient subgroups vs vemurafeniba,7
For complete information, please refer to the Summaries of Product Characteristics.